“This finding could change the way we look at phase transformations within the cathode and the resulting loss of capacity in this class of material,” said Alpesh Khushalchand Shukla, a scientist at Berkeley Lab’s Molecular Foundry, and lead author of the study. “Our work shows that it is extremely important to completely characterize a new material in its pristine state, as well as after cycling, in order to avoid misinterpretations.”
Previous work by researchers at the Molecular Foundry, a research center specializing in nanoscale science, revealed the structure of cathode materials containing “excess” lithium, resolving a longstanding debate.
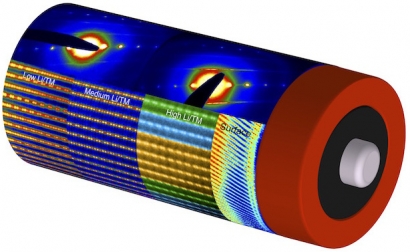
Using a suite of electron microscopes both at the National Center for Electron Microscopy, a Molecular Foundry facility, and at SuperSTEM, the National Research Facility for Advanced Electron Microscopy in Daresbury, U.K., the research team found that while the atoms throughout the interior of the cathode material remained in the same structural pattern across all compositions, decreasing the amount of lithium caused an increase in randomness in the position of certain atoms within the structure.
By comparing different compositions of cathode material to battery performance, the researchers demonstrated it was possible to optimize battery performance in relation to capacity by using a lower ratio of lithium to other metals.
Berkeley Lab’s Molecular Foundry is a DOE Office of Science User Facility.
This work was supported by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy, Office of Basic Science, and Small Business Voucher Pilot Program; Envia Systems; and the U.K.’s Engineering and Physical Science Research Council.
The work was published in the journal Energy & Environmental Science.
Illustration: Atomic resolution scanning transmission electron microscopy images and electron diffraction patterns, arranged on a rendering of a battery, show how the structure of lithium-rich and manganese-rich transition metal oxides used inside battery cathodes changes with composition. The images also show how the surface of the cathode has a different structure than the interior. (Credit: Berkeley Lab)

