Hydrogen is a clean fuel currently used to propel rockets in NASA’s space program and is widely expected to play an important role in a sustainable energy future. Water electrolysis using electricity from solar PV offers a promising route to produce H2 without any associated CO2 emissions.
Esposito’s team has now developed a novel photovoltaic-powered electrolysis device that can operate as a stand-alone floating platform. His floating PV-electrolyzer can be thought of as a “solar fuels rig” that bears some resemblance to deep-sea oil rigs, except that it would produce hydrogen fuel from sunlight and water instead of extracting petroleum from beneath the sea floor. The study (DOI: 10.1016/j.ijhydene.2017.11.086), “Floating Membraneless PV-Electrolyzer Based on Buoyancy-Driven Product Separation,” was published recently by International Journal of Hydrogen Energy.
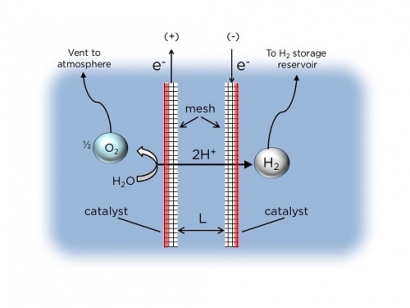
The researchers’ key innovation is the method by which they separate the H2 and O2 gases produced by water electrolysis. The Columbia Engineering device relies on a novel electrode configuration that allows the gases to be separated and collected using the buoyancy of bubbles in water. The design enables efficient operation with high product purity and without actively pumping the electrolyte. Based on the concept of buoyancy-induced separation, the simple electrolyzer architecture produces H2 with purity as high as 99 percent.
“The simplicity of our PV-electrolyzer architecture—without a membrane or pumps—makes our design particularly attractive for its application to seawater electrolysis, thanks to its potential for low cost and higher durability compared to current devices that contain membranes,” says Esposito, whose Solar Fuels Engineering Laboratory develops solar and electrochemical technologies that convert renewable and abundant solar energy into storable chemical fuels. “We believe that our prototype is the first demonstration of a practical membraneless floating PV-electrolyzer system, and could inspire large-scale ‘solar fuels rigs’ that could generate large quantities of H2 fuel from abundant sunlight and seawater without taking up any space on land or competing with fresh water for agricultural uses.”
“Being able to safely demonstrate a device that can perform electrolysis without a membrane brings us another step closer to making seawater electrolysis possible,” says Jack Davis, the paper’s first author and a PhD student working with Esposito.
Crucial to the operation of Esposito’s PV-electrolyzer is the mesh flow-through electrodes that are coated with a catalyst only on one side. These asymmetric electrodes promote the evolution of gaseous H2 and O2 products on only the outer surfaces of the electrodes where the catalysts have been deposited. When the growing H2 and O2 bubbles become large enough, their buoyancy causes them to detach from the electrode surfaces and float upwards into separate overhead collection chambers.
The team is refining their design for more efficient operation in real seawater, which poses additional challenges compared to the more ideal aqueous electrolytes used in their laboratory studies. They also plan to develop modular designs that they can use to build larger, scaled-up systems.
Esposito concluded, “We are especially excited about the potential of solar fuels technologies because of the tremendous amount of solar energy that is available. Our challenge is to find scalable and economical technologies that convert sunlight into a useful form of energy that can also be stored for times when the sun is not shining.”
The study is titled “Floating Membraneless PV-Electrolyzer Based on Buoyancy-Driven Product Separation.”
Authors include: Jonathan Davis, Ji Qi, Xinran Fan, Justin Bui, and Daniel V. Esposito, all in the department of chemical engineering, Columbia Engineering.
The study was funded by Columbia University start-up funds.
Illustration: Schematic side-view of passive membraneless electrolysis. Two mesh electrodes are held at a narrow separation distance (L), and generate H2 and O2 gases concurrently. The key innovation is the asymmetric placement of the catalyst on the outward facing surfaces of the mesh, such that the generation of bubbles is constrained to this region. When the gas bubbles detach, their buoyancy causes them to float upward into separate collection chambers. (Daniel Esposito/Columbia Engineering)

